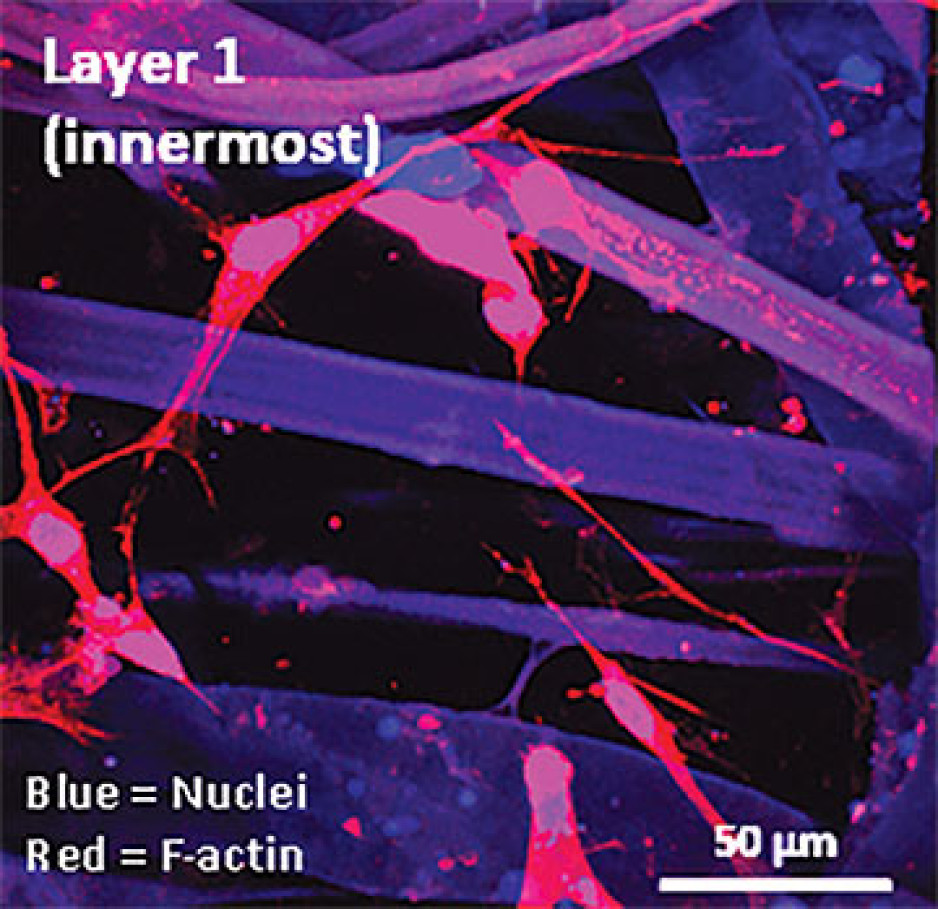
When challenged by surgeons to find better treatments for difficult-to-manage connective tissue diseases, Dr. David O’Gorman gladly accepted.
Dr. O’Gorman is a Molecular Biologist and Lawson Scientist based at St. Joseph’s Hospital, a part of St. Joseph’s Health Care London. His research focuses on understanding normal and abnormal connective tissue repair. He collaborates with researchers and clinicians working in many different disciplines, including those specializing in reconstructive surgery, orthopedics and urology.
Surgical reconstructions can be hampered by a lack of graft tissue, or graft tissue of insufficient quality, making it difficult to achieve optimal outcomes for the patients.
An example is a condition called urethral stricture disease (urethral scarring). This condition occurs in males and typically causes symptoms such as frequent and urgent urination, and slow urinary stream. In extreme cases, it can cause urinary tract infections, permanent bladder dysfunction and renal failure. Recurrence rates after minimally invasive treatments are high, and so many urologists recommend open surgical approaches.
Surgeons can use the patient’s own tissues to reconstruct the urethra after stricture removal. This tissue is normally sourced from the buccal cavity in the mouth but taking large tissue grafts can result in complications. In cases where buccal grafts have been used for previous reconstructions, there may not be enough intact tissue left.
Dr. O’Gorman sees a solution in growing sheets of human buccal tissues in the lab.
“We are currently using buccal graft trimmings as a source of cells, culturing them in a 3D environment and expanding them to create tissues of suitable size, density and elasticity.”
The patient’s own cells are used to generate a tissue graft for urethral reconstruction. While several research groups have developed this approach in the past, few have attempted to translate their models for clinical use.
“Our immediate goal is to provide proof of principle – that we can consistently generate grafts of suitable size and functional characteristics,” explains Dr. O’Gorman, “In the future, we could be providing bioengineered graft tissues for reconstructive surgeries here in London.”
Bioengineered human tissues can also be used as ‘mimetics’ – replications of human tissues – to study diseases, especially those difficult to model using routine laboratory methods.
Instead of a using a growth media or sterile plastic dishes, 3D cell culture is achieved by embedding cells in a matrix of proteins and other molecules normally found in those tissues. In this environment, gene expression and growth is more similar to cells of connective tissues in the body being replicated.
Dupuytren’s disease (or Dupuytren’s Contracture) affects the palmar fascia in the hand, a connective tissue beneath the skin that extends from the base of the palm into the fingers. This disease can be understood as a type of excessive scarring, where normal tissue repair processes have gone awry and dense scar tissue forms, typically causing permanent palm or finger flexion that restricts hand function.
This condition is surprisingly common and may affect more than one million people in Canada. While there are surgical treatment options available, none consistently prevent this disease from recurring in at least a third of patients.
“Due to its high recurrence rate after treatment, Dupuytren’s disease is currently considered incurable. Our challenge is to understand it well enough to develop truly effective treatments,” says Dr. O’Gorman.
Human hands have unique characteristics not found in other species, making animal models impractical. Instead, Dr. O’Gorman’s team extracts cells from the diseased palmar fascia of patients undergoing hand surgeries and bioengineers them into palmar fascia ‘contractures’ in the lab.
“Since the cells from a single palmar fascia sample can be used to grow dozens of little contractures, we can test many different treatments simultaneously to see what works best for each patient.”
This approach may also allow them to determine if Dupuytren’s disease is truly one disease, or a group of similar diseases that cause palm and finger contractures.
“Often, Dupuytren’s disease is clearly heritable, but some individuals have no family history of it and develop apparently sporadic disease,” notes Dr. O’Gorman. “We want to determine if these are truly the same disease at the molecular level.”
Another major cause of abnormal connective tissue repair is infection, and tissue mimetics can play a role here, too. While rare, infections of artificial joint replacements are particularly devastating for patients, as they typically require readmission to hospital to remove the infected joint, weeks of antibiotic-based treatment, and an additional surgery to replace the artificial joint.
In addition to the associated pain and suffering, these procedures are technically challenging and costly to our health care system.
Artificial shoulder joint infections are most frequently caused by the microorganism Cutibacterium acnes (C. acnes). C. acnes infections disrupt normal tissue repair processes after surgery, cause shoulder tissues to die and promote loosening of the artificial joint. These infections are difficult to diagnose, and there is a lack of reproducible
models in which to study them. Dr O’Gorman’s team has set out to create the first human Shoulder-Joint Implant Mimetic (S-JIM) of C. acnes infection.
“While S-JIMs are more complex, they are 3D in vitro cell culture systems designed to mimic human tissues, like those that we use for studying Dupuytren’s disease.”
S-JIMs include layers of artificial human tissue, wrapped around cores of titanium alloy or cobalt chrome, the metals used to create artificial joints. They are co-cultured with C. acnes under low oxygen conditions similar to those that normally occur around artificial shoulder joints.
“We are bioengineering simple 3D cell cultures to more closely mimic the complexity of human tissues, with blood supply, nerves and interactions with other cells.” – Dr. David O’Gorman
Studying the connective tissue layers close to the infection allows researchers to investigate processes that promote infection, such as the formation of a biofilm that harbours and protects the bacteria from the body’s immune system. They are also able to test whether novel treatments can disrupt biofilm formation and increase the effectiveness of antibiotics.
Dr. O’Gorman predicts that in the future, medical researchers will routinely use bioengineered 3D human tissue and organ mimetics to accelerate our understanding of disease.
“The technology is in its infancy, but the potential for using bioengineered human tissues for surgical reconstructions or as disease models is huge. At Lawson, we’re ready to take on health care challenges and build on innovative approaches to improve the quality of life for patients.”
ONLINE EXCLUSIVE: What is 3D cell culture?
Medical researchers have grown human cells in culture media on or in sterile plastic dishes, such as Petri dishes, for more than 50 years.
Some cells, such as blood cells, can survive and grow in suspension, while others like smooth muscle cells need¬ to adhere to a surface to survive and grow. These are often called “2D cell cultures” because the cells grow horizontally across the bottom of the dish.
Some cells derived from connective tissues, such as fibroblasts, are not only adherent, but also very sensitive to the stiffness of their environment (“biomechanically sensitive” cells). Plastic dishes are at least 10,000 times stiffer than most connective tissues, and when biomechanically sensitive cells detect stiff surfaces, they can change the expression of their genes and behave abnormally.
The most common proteins in these tissues - and in the entire human body - are collagens, and one routine 3D cell culture approach is to embed fibroblasts in a collagen gel (gelatin). Fibroblasts in this environment can grow in any direction they choose, and their gene expression is more similar to cells in connective tissues.
These simple 3D cell cultures represent tissue engineering in its most basic form.
“Our challenge is to bioengineer simple 3D cell cultures in the lab to more closely mimic the complexity of human tissues, which have blood supply, nerves and interactions with other cells and tissues that modify their function and ability to heal after injury,” explains Dr. O’Gorman.
Dr. David O’Gorman is a Lawson Scientist and Co-director, Cell and Molecular Biology Laboratory at The Roth | McFarlane Hand and Upper Limb Centre in London, Ontario. He is also an Assistant Professor at Western University.