LONDON, ON- A new diagnostic method could help identify one of the deadliest types of interstitial lung disease (ILD) sooner, allowing for faster treatment and improved patient outcomes.
Idiopathic pulmonary fibrosis (IPF) is one of the most serious and common types of ILD, occurring most often in patients 60 and older with an average survival time of three to five years. At any given time roughly 300 patients are being treated for IPF in London, Ontario. Globally, it is the number one reason for lung transplants.
A new study published in Respiratory Research has found that testing for a protein called cyclin-dependent kinase inhibitor protein (p16) in biopsy tissue may help more accurately identify IPF.
Dr. Marco Mura, Associate Scientist at Lawson and Respirologist at London Health Sciences Centre (LHSC), together with Dr. Matthew J. Cecchini, Pathologist at LHSC, led the study. Dr. Mura says the test could mean knowing years sooner if a lung transplant might be needed.
“We developed a method that is actually quite inexpensive to increase the diagnostic accuracy of the biopsy and help to avoid unclassifiable cases. The method has a prognostic value, so it helps predict survival of these patients at the time of biopsy,” says Dr. Mura, who is also an Associate Professor of Medicine at Western University.
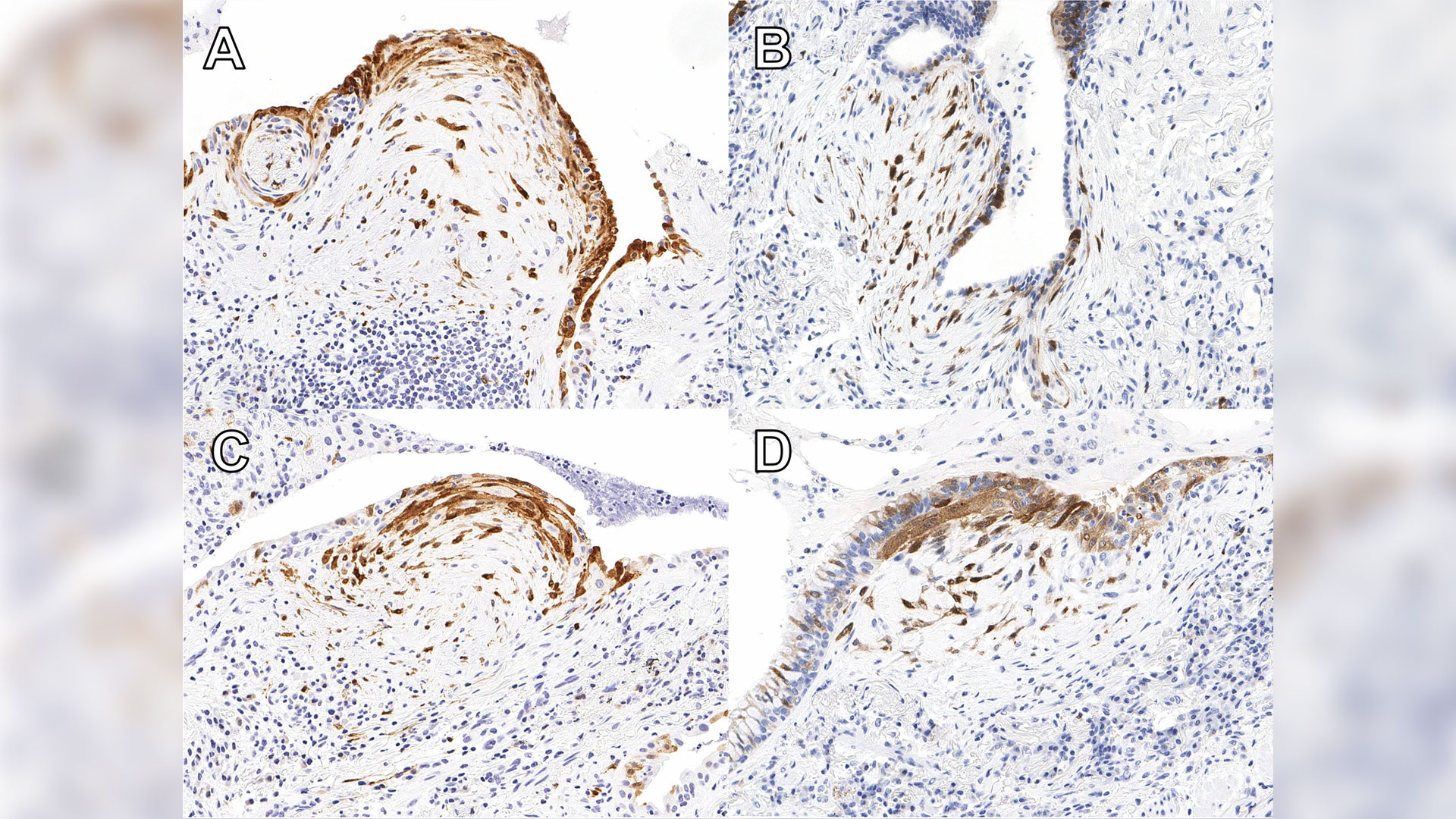
Patients with high expression of p16 were shown to experience worse outcomes than others with ILD, indicating that it is essential for these patients to start necessary treatment without delay. The protein is already widely used in ovarian cancer diagnosis. Now additional ongoing studies will help reinforce the value of the test for IPF diagnosis.
“We have no tests that we can apply to the (lung) biopsy other than the pathologist looking at it and saying ‘OK, this biopsy shows this pattern,’” Dr. Mura says. “There were absolutely zero additional biomarker tests to reinforce, validate or support the diagnosis. So, this will be the first time that we implement such test biomarkers in clinical practice.”
With this type of test that looks at the quantity of the biomarker, there is also the possibility of applying artificial intelligence to advance diagnosis in the future.
The research was supported by a 2018 Internal Research Fund grant from Lawson.
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Communications Consultant & External Relations
Lawson Health Research Institute
T: 519-685-8500 ext. ext. 64059
C: 226-919-4748
@email


